Encyclopedia of Earth
Encyclopedia of Earth: Molybdenum
Information about the metallic element, Molybdenum, atomic number 42. It is unique in having an extremely high melting point. Covers its discovery, its biological importance, its physical and atomic properties, how abundant it is on the...
Encyclopedia of Earth
Encyclopedia of Earth: Mendelevium
Information about the synthetic, radioactive element, Mendelevium, atomic number 101. Covers its discovery, physical and atomic properties, and details about permissible exposure.
Encyclopedia of Earth
Encyclopedia of Earth: Manganese
Information about the element, Manganese, atomic number 25. Covers physical properties, atomic properties, how abundant it is on the Earth, details about health-related regulations, and its importance to human health. Also discusses...
Other
Chemical elements.com/scandium
A site containing a good deal of elemental information. Information on each element includes atomic number, atomic mass, melting point, boiling point, density, isotopes and more.
TeachEngineering
Teach Engineering: Mixtures and Solutions
This unit covers introductory concepts of mixtures and solutions. Students think about how mixtures and solutions, and atoms and molecules can influence new technologies developed by engineers. The first lesson explores the fundamentals...
Texas Instruments
Texas Instruments: Periodicity of the Periodic Table
Different properties of the elements of the periodic table will be plotted and from this, a system of organization for the elements will be developed.
US Environmental Protection Agency
Epa: Mercury
This site provides a user navigated slide show with animations providing basic information about the element Mercury.
Science Education Resource Center at Carleton College
Serc: Building 3 D Models of Atoms
Learners build atoms of elements 1-20. The entire set can be hung from the ceiling in order of the Periodic Table to be referred to throughout the Chemistry class/unit. This is helpful when teaching bonding and patterns of the Periodic...
Royal Society of Chemistry
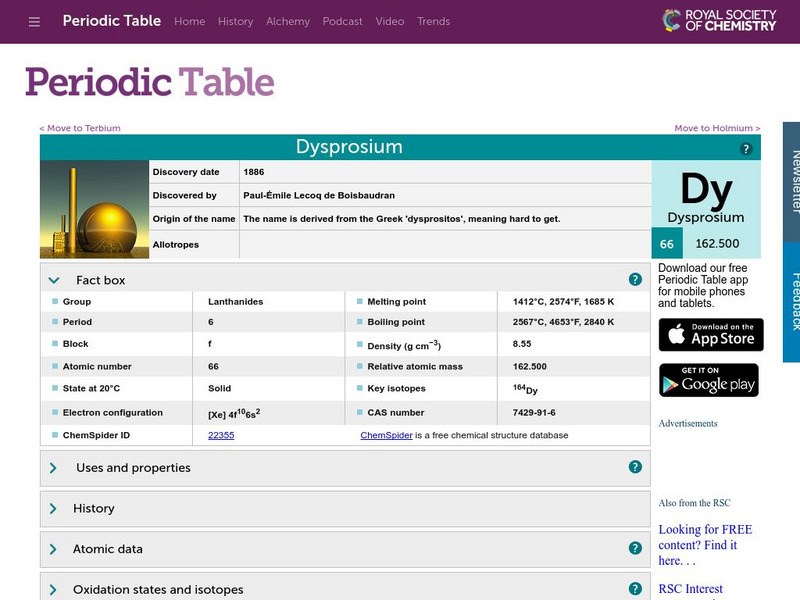
Visual Elements: Dysprosium
Brief summary of uses and lack of uses for this element.
Royal Society of Chemistry
Visual Elements/history
A history and description of the modern periodic table, including its early origins, many contributors and later additions.
Other
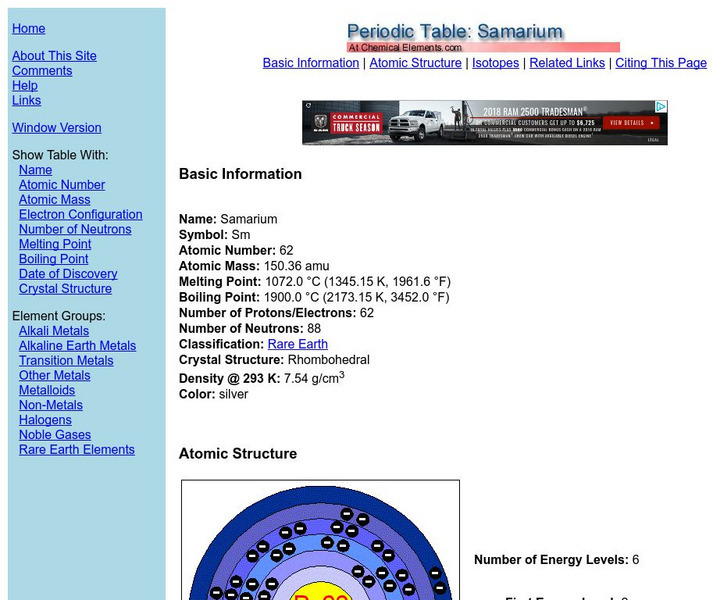
Chemical elements.com: Samarium
A site containing a good deal of elemental information. Information on each element includes atomic number, atomic mass, melting point, boiling point, density, isotopes and more.
Other
Chemical elements.com: Silver
A site containing a good deal of elemental information. Information on each element includes atomic number, atomic mass, melting point, boiling point, density, isotopes and more.
Other
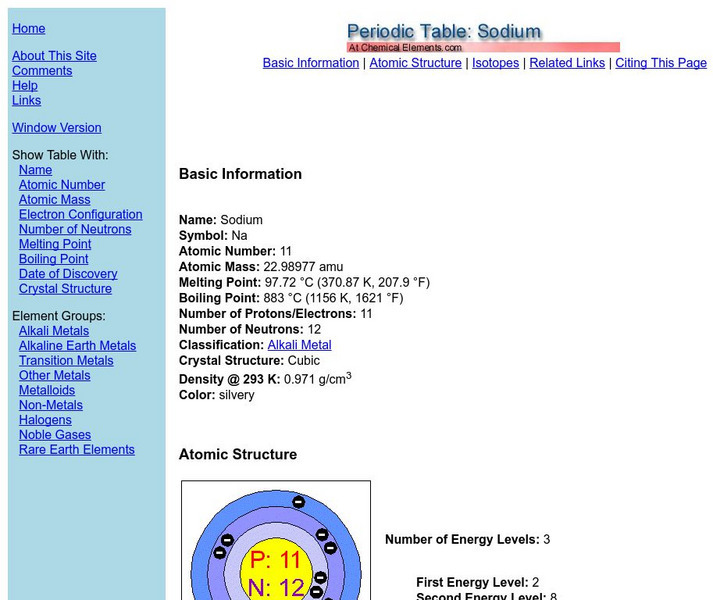
Chemical elements.com: Sodium
A site containing a good deal of elemental information. Information on each element includes atomic number, atomic mass, melting point, boiling point, density, isotopes and more.
Web Elements
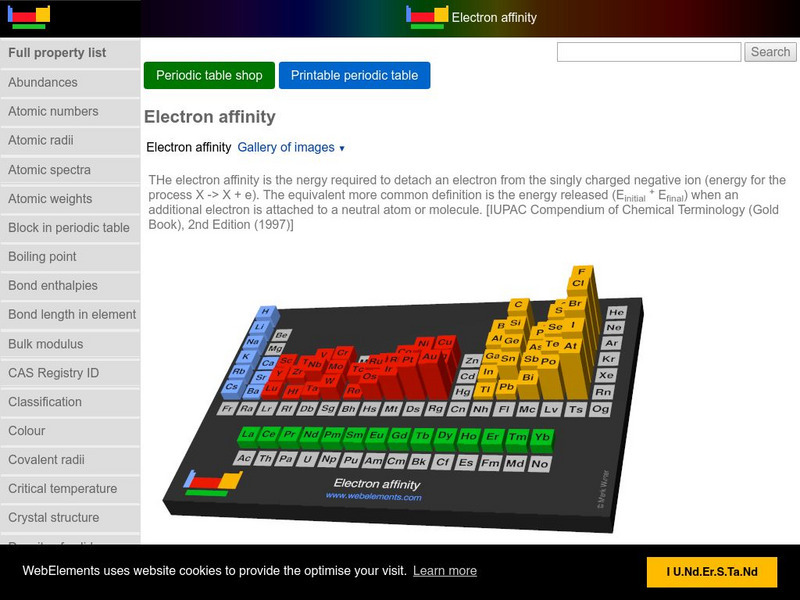
Web Elements Periodic Table: Electron Affinity
An interactive periodic table. When you hover over an element, its name, atomic number, group, period, and electron affinity value appear.
Alabama Learning Exchange
Alex: Periodi Cally Used Elements
This lesson explores the various uses of elements in the periodic table. Students will be assigned an element to research. They will then present its uses and atomic information to the class.
Georgia State University
Georgia State University: Hyper Physics: Ionization Energies
A concise description of ionization energy, and a line graph that plots the ionization energies of the first 88 elements vs. the atomic numbers.