Curated OER
Development of the Atomic Theory
In this atomic theory worksheet, learners complete a paragraph describing the development of the atomic theory by filling in the blank with 21 terms.
Curated OER
Nuclear Scientists Project
Students explore nuclear scientists. In this nuclear science research lesson, students choose a scientist who has contributed to nuclear theory, research his/her life and accomplishments, and write a paper. Students generate...
Curated OER
Bonding Theories
In this bonding worksheet, students fill in 8 blanks with the appropriate terms about theories of bonding, they determine if 6 statements are true or false, they match 5 terms with their meanings and they solve 1 problem related to...
Curated OER
Historical Development of a Scientific Idea
Ninth graders examine how scientist's contribute to atomic theory. In this development of a scientific idea lesson students work in groups and research the development of the atomic model.
Curated OER
Subatomic Particles
For this atoms worksheet, students complete a graphic organizer comparing the properties of the 3 subatomic particles. Then students complete 1 short answer question.
Curated OER
Practice Final
A full-fledged practice final prepares pupils for their general chemistry final exam. If they complete these 57 multiple-choice questions correctly, they will be well-prepared. Note: even though the questions are multiple-choice, there...
Curated OER
A Model of a Scanning Tunneling Microscope
Ninth graders explain how a scanning tunneling microscope works. In this chemistry lesson, 9th graders construct atomic models and simulate how their images appear under the STM. They discuss the limitations of their atomic model.
Curated OER
Matter and Energy
Students participate in a small group read aloud of the short story, "Cerium" by Primo Levi. They answer several questions about the story and then relate the reading to a lecture on Kinetic theory. After the lecture they apply the...
Curated OER
Ionic bonding
Students explore ionic bonding. They draw examples of ionic bonding and explain the activities of the electrons of the elements. Students use paper plates and candy to draw electron configurations of given atoms.
Curated OER
States of Matter
Students explore the states of matter. They discuss the different phrases of matter and categorize everyday substances as solids, liquids, or gases. Students explore the relationship between the phases on an atomic level and the role of...
Curated OER
Chemistry & The Community
Students complete a Webquest which investigates the chemistry in items such as shampoo. They research the Internet, perform a lab experiment, and write a scientific lab report with their findings. Upon completion of the activities, the...
Curated OER
Fermi Observatory Measures the Lumps in Space
In this gamma-ray instructional activity, students read about the Fermi Gamma-ray Observatory and how it measures the invisible lumps in space. Students solve 3 problems using an equation to determine the time that gamma-rays travel in...
Curated OER
An Ad for an Element
Students prepare an ad for an element, including properties and uses, in an attention getting format such as that used in the advertising business.
Curated OER
Quantum Physics
Students discuss the mass-energy relationship based on Einstein's work. They calculate the energy released in various scenerios and sketch diagrams for the Lyman, Balmer and Pfund Series. In groups, they discuss the role of photons and...
Curated OER
Causal Patterns in Density Phenomena
Students consider the causes of density at a microscopic level. They then discover that one cause of density has to do with how many protons and neutrons the material contains.
Curated OER
States of Matter
Students categorize items into one of the three states of matter. They participate in a demonstration in which they represent particles of matter. Finally, they complete an experiment in which they "race" top see who can make acetone...
CK-12 Foundation
Ck 12: Atomic Theory
[Free Registration/Login may be required to access all resource tools.] In this online tutorial students will explain the law of conservation of mass, the law of definite proportions, and the law of multiple proportions. They will also...
National High Magnetic Field Laboratory
Magnet Academy: Paul Dirac
Paul Adrien Maurice Dirac was an outstanding twentieth century theoretical physicist whose work was fundamental to the development of quantum mechanics and quantum electrodynamics. He was awarded the Nobel Prize for Physics jointly with...
TeachEngineering
Teach Engineering: Mixtures and Solutions
This unit covers introductory concepts of mixtures and solutions. Students think about how mixtures and solutions, and atoms and molecules can influence new technologies developed by engineers. The first lesson explores the fundamentals...
University of Houston
University of Houston: Engines of Our Ingenuity: John Dalton's Notation
This is part of a small radio show at the University of Houston. It talks about how John Dalton came up with his version of chemical notation, and how it differs from our version of it today. It is available in audio form also.
National High Magnetic Field Laboratory
Magnet Academy: Enrico Fermi
Enrico Fermi was a titan of twentieth-century physics. He outlined the statistical laws that govern the behavior of particles that abide by the Pauli exclusion principle and developed a theoretical model of the atom in his mid-twenties....
Lawrence Berkeley National Laboratory
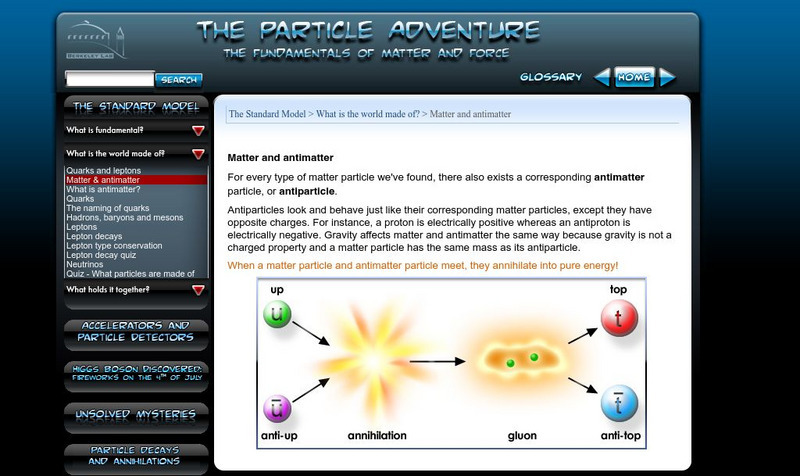
Berkeley Lab: Particle Adventure: Matter and Antimatter
The beginning of an informative tutorial on antimatter, covering quarks, hadrons, baryons, mesons, leptons, and neutrinos.
Wikimedia
Wikipedia: Ferromagnetism
This site from Wikipedia provides a wonderful in-depth explanation of ferromagnetism, covering the atomic behavior which is responsible for ferromagnetic properties. Also introduces the concepts of magnetic domains and the Curie...
Concord Consortium
The Molecular Workbench Database: Models of the Atom's Electron Orbitals
Learn about atomic structure and the multiple theories of atomic structure in this simulation.